Parenteral drug administration using prefilled single-dose syringes has the potential to add ease and safety for treating a variety of diseases, including autoimmune, arthritis, diabetes, and others. Using pre-filled syringes presents the ability to reduce medical errors and enable patient-controlled self-therapy.
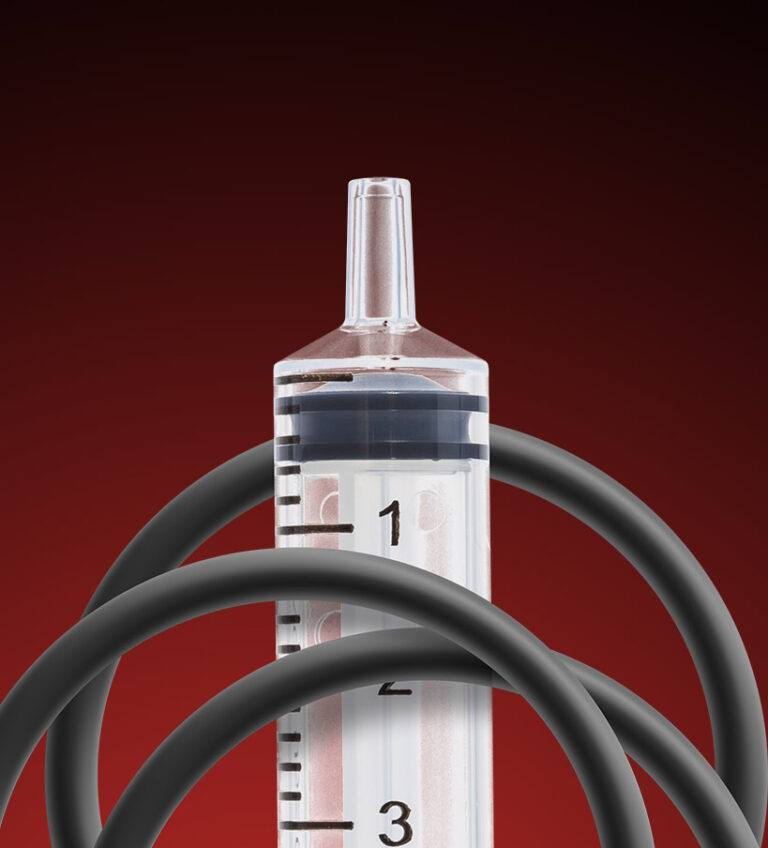
However, the traditional method of coating single-dose syringes with silicone oil may pose additional complications. Mainly, the inner and outer wall coating of a syringe with silicone oil during manufacturing can result in denaturing of the therapeutics. For typical storage lifetimes, silicone oil has been shown to induce conformational changes and aggregation of protein-based therapeutics. Moreover, silicone oils have also been known to contract and form micro-droplets that may even migrate into the drug solution. This migration of silicone oil can potentially cause serious complications.
Recognizing the need, GVD responded to an SBIR to innovate a coating solution that is able to reduce the force required to move the rubber stopper through the syringe as well or better than silicone oil, without the risk of affecting the solution inside. The results showed that GVD’s PTFE coating, which is chemically inert, was lubricious and provided adequate friction reduction for ideal operation of a single-use syringe. Unlike silicone oil, GVD’s PTFE coating does not breakdown or cause denaturing of therapeutics within a syringe.
Also, GVD’s PTFE coating is conformal in 3D, which overcomes challenges of common liquid phase coating methods that lack conformality in three dimensions. Lastly, GVD’s PTFE coating is a low temperature coating process that does not degrade the rubber stopper materials within a syringe.